Illuminating the Luminescent Luciferins
- Behind The Scalpel

- Jun 9, 2020
- 3 min read
Luciferins are a group of organic molecules that glow, causing bioluminescence found underwater, in the air and in caves. Bioluminescent plankton, fireflies, glow worms and jellyfish all glow when their Luciferins are activated, all for very different reasons. The chemistry is similar an all, which I hope to explain now.
Bioluminescence is a type on chemiluminescence, but found naturally in living organisms. Some organisms produce their own molecules responsible for the light emitted, and some obtain it from feeding and accumulation of the nessasary molecules.
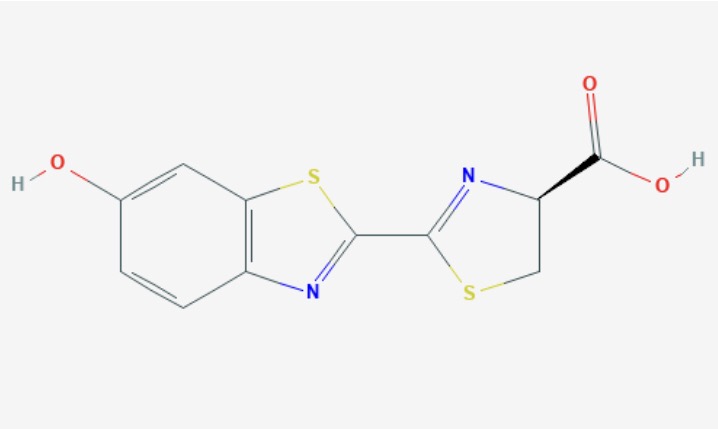
To explain the mechanism of the glow, I am going to focus on the most understood Luciferin, Firefly Luciferin or, of you prefer, its very long IUPAC name is (4S)-2-(6-hydroxy-1,3-benzothiazol-2-yl)-4,5-dihydro-1,3-thiazole-4-carboxylic acid.
Firefly Luciferin is found as an racemic with two enantiomers, D-Lucferin and L-Luciferin. Both have been found to produce light, however the D-Luciferin more so.
The mechanism to produce light has many stages including ATP and oxygen and is helped by many catalysts and reagents. The first stage forms Luciferyl Adelyate, or Luciferyl-AMP from the luciferin molecule. Here, a molecule of ATP is used, with Adenosine monophosphate (AMP) binding with the luciferin, and 2 phosphate ions being released. A proton (H+) is then removed from the Carbon-4, with the help of the enzyme Luciferase. The anion formed binds with atmospheric oxygen at this negative carbon. This forms a very reactive intermediate which quickly goes on to form the oxidised luciferin molecule, CO2 and an AMP molecule. This oxidised luciferin, or oxyluciferin, is in an excited state, meaning it releases light at this point.
The excited ketone is the generally accepted form for firefly luciferin, but there are other forms thought to be present in the light production, mostly dependant on the pH during the reaction.
In the diagram showing these reactions, an asterix(*) shows where a molecule is in its excited state and the use of a wavy line indicates where the not so important part of the molecule has been left out in each diagram.
The excited state of the ketone produces photons of light. This occurs when he excited outer electron, which has been elevated up to a higher energy orbit falls back down to its original orbit and the energy difference between these two orbits is emitted as light. The molecule has then returned to what is know as its ground state.
The same idea is used in crime scenes to find remains of blood after it has been cleaned up. The luminol used also produces light by a very similar mechanism, however, instead of Oxygen, Iron is needed. Therefore, when luminol comes into contact with Iron from haemoglobin in the blood, the distinctive blue glow is produced.
This chemistry is also now being used in genetic research and drug research, as the gene for the production of luciferin can be added into the gene or pathway being researched. Then wherever and whenever the protein is expressed or pathway activated, it is seen due to the glow from the luciferin also being present. This is a non toxic way of researching on living organisms, without requiring their death, seen in the mice below. The most common example of this is in cancer research, as the glow can show easily the mass and location of the tumor. Therefore, the effectivness of a anti-tumor drug can be easily assessed in pre-clinical trials.

References
Evers, J., 2013.Bioluminescence. [online] National Geographic Society. Available at: <https://www.nationalgeographic.org/encyclopedia/bioluminescence/> [Accessed 2 June 2020].
National Center for Biotechnology Information. PubChem Database. L-Luciferin, CID=135750019, https://pubchem.ncbi.nlm.nih.gov/compound/L-Luciferin#section=Names-and-Identifiersl (accessed on June 2, 2020)
Brachini, B., 2013.CHEMISTRY OF FIREFLY BIOLUMINESCENCE. [online] Photobiology.info. Available at: <http://photobiology.info/Branchini2.html> [Accessed 28 May 2020].
Prescher JA, Contag CH. Guided by the light: visualizing biomolecular processes in living animals with bioluminescence.Curr Opin Chem Biol. 2010;14(1):80‐89. doi:10.1016/j.cbpa.2009.11.001
Dothager, Robin S et al. “Advances in bioluminescence imaging of live animal models.”Current opinion in biotechnologyvol. 20,1 (2009): 45-53. doi:10.1016/j.copbio.2009.01.007
Figure 1 (Firefly Luciferin Molecule) National Center for Biotechnology Information. PubChem Database. L-Luciferin, CID=135750019, https://pubchem.ncbi.nlm.nih.gov/compound/L-Luciferin#section=Names-and-Identifiersl (accessed on June 2, 2020)
Figure 2 (Firefly Luciferin Mechanism) Brachini, B., 2013.CHEMISTRY OF FIREFLY BIOLUMINESCENCE. [online] Photobiology.info. Available at: <http://photobiology.info/Branchini2.html> [Accessed 28 May 2020].
Figure 3 (Bioluminesence used in Rodents) Evers, J., 2013.Bioluminescence. [online] National Geographic Society. Available at: <https://www.nationalgeographic.org/encyclopedia/bioluminescence/> [Accessed 2 June 2020].






Comments